產品櫥窗
-
Sodium Bicarbonate, NaHCO3, >99%
|
產品型號:101-144-55-8 商品規格: |
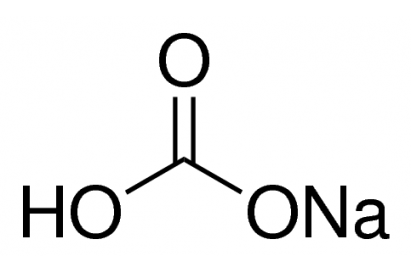 |
Synonym: Sodium hydrogen carbonate
NaHCO3
M.W.: 84.01
Sodium bicarbonate may be used to remove or wash acidic impurities from a crude liquid to yield a purer sample. Sodium bicarbonate reacts with the carboxyl groups to give a fast-forming and effervescent CO2 formation which has been used to test for the presence of carboxylic groups in proteins.
Store at Room Temperature.