產品櫥窗
-
Aprotinin
|
產品型號:101-9087-70-1 商品規格: |
 |
Serine Protease Inhibitor. Synonym: Trasylol, Trypsin inhibitor (basic)
C284H432N84O79S7
M.W.: 6511.44
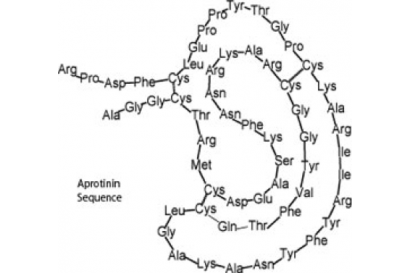
Sequences: RPDFCLEPPYTGPCKARIIRYFYNAKAGLCQTFVYGGCRAKRNNFKSAEDCMRTCGGA
Appearance: White Solid
Purity: One single band (Electrophoresis)
Solubility: Soluble in H20 (10mg/ml)
Trypsin inhibitor which inhibits kallikrein, trypsin, chymotrypsin, and various serine proteases.
Aprotinin is a competitive serine protease inhibitor that forms stable complexes with and blocks the active sites of enzyme. This binding is reversible, and most aprotinin-protease complexes will dissociate at extreme pH levels >10 or <3. Structurally, Aprotinin is a monomeric globular protein derived from bovine lung that consists of 58 amino acids, arranged in a single polypeptide chain with three crosslinking disulfide bridges.
Source: Bovine lungs sourced from licensed slaughterhouses in New Zealand. The lungs have passed both pre and post-mortem inspection and are approved for pharmaceutical use by Government Veterinary inspectors in accordance with New Zealand law.
Aprotinin is freely soluble in water (>10 mg/mL) and in aqueous buffers of low ionic strengths.5,6 Dilute solutions are generally less stable than concentrated ones. Solution stability also depends on pH; values of 1-12 can be tolerated.4 Repeated freeze-thaw cycles should be avoided. The Cys14-Cys38 disulfide bridge is readily split by reducing agents like β-mercaptoethanol.4 Due to its compact tertiary structure, aprotinin is relatively stable against denaturation due to high temperature, acids, alkalies, organic solvents or proteolytic degradation (only thermolysin has been found capable of degrading aprotinin after heating to 60-80°C).4 The high basicity of aprotinin causes it to adhere to commonly used dialysis tubing and even gel filtration matrices, but the use of acetylaated materials and concentrated salt solutions (e.g., ≥0.1 M NaCl in buffer) minimizes the problem.4 Sterilization may be achieved by filtration through a 0.2 μm filter.
M.W.: 6511.44
Sequences: RPDFCLEPPYTGPCKARIIRYFYNAKAGLCQTFVYGGCRAKRNNFKSAEDCMRTCGGA
Appearance: White Solid
Purity: One single band (Electrophoresis)
Solubility: Soluble in H20 (10mg/ml)
Trypsin inhibitor which inhibits kallikrein, trypsin, chymotrypsin, and various serine proteases.
Aprotinin is a competitive serine protease inhibitor that forms stable complexes with and blocks the active sites of enzyme. This binding is reversible, and most aprotinin-protease complexes will dissociate at extreme pH levels >10 or <3. Structurally, Aprotinin is a monomeric globular protein derived from bovine lung that consists of 58 amino acids, arranged in a single polypeptide chain with three crosslinking disulfide bridges.
Source: Bovine lungs sourced from licensed slaughterhouses in New Zealand. The lungs have passed both pre and post-mortem inspection and are approved for pharmaceutical use by Government Veterinary inspectors in accordance with New Zealand law.
Aprotinin is freely soluble in water (>10 mg/mL) and in aqueous buffers of low ionic strengths.5,6 Dilute solutions are generally less stable than concentrated ones. Solution stability also depends on pH; values of 1-12 can be tolerated.4 Repeated freeze-thaw cycles should be avoided. The Cys14-Cys38 disulfide bridge is readily split by reducing agents like β-mercaptoethanol.4 Due to its compact tertiary structure, aprotinin is relatively stable against denaturation due to high temperature, acids, alkalies, organic solvents or proteolytic degradation (only thermolysin has been found capable of degrading aprotinin after heating to 60-80°C).4 The high basicity of aprotinin causes it to adhere to commonly used dialysis tubing and even gel filtration matrices, but the use of acetylaated materials and concentrated salt solutions (e.g., ≥0.1 M NaCl in buffer) minimizes the problem.4 Sterilization may be achieved by filtration through a 0.2 μm filter.
Store at 4℃.
Shipping at Room Temp within Taiwan; may vary elsewhere
Shipping at Room Temp within Taiwan; may vary elsewhere