產品櫥窗
-
Cefepime hydrochloride(Stock: Inquire)
|
產品型號:101-123171-59-5 商品規格: |
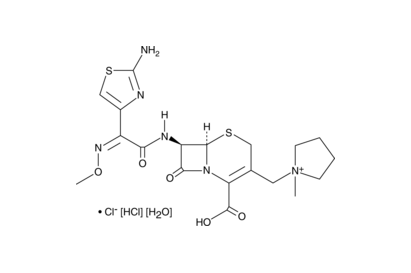 |
Cefepime dihydrochloride monohydrate
C19H28Cl2N6O6S2
MW: 571.50
Purity: >98%
Cefepime is a fourth generation cephalosporin antibiotic effective against gram-negative and gram-positive bacteria. It is resistant to a variety of β-lactamases, and is effective against some naturally resistant bacterial species such as Pseudomonas aeruginosa, Staphylococcus aureus, and Streptococcus pneumoniae. Cefepime HCl is more soluble compared to the pure Cefepime. Both forms have similar potencies and both are suitable for microbiology use. However, Cefepime HCl can be easier to work with because of its higher aqueous solubility.
Cephalosporins are a type of β-lactam antibiotic consisting of a four-membered β-lactam ring bound to a six-membered dihydrothiazine ring. This two-ring system causes distortion of the β-lactam amide bond, resulting in decreased resonance stabilization and increased reactivity. β-lactams inhibit the formation of peptidoglycan cross-links within bacterial cell walls by targeting penicillin-binding proteins or PBPs. Consequently, the bacterial cell wall becomes weak and cytolysis occurs. Cephalosporins are less susceptible to β-lactamases than the penicillin β-lactam antibiotics.
Stock conc.: 10mg/mL, in PBS. Use immediately.
MW: 571.50
Purity: >98%
Cefepime is a fourth generation cephalosporin antibiotic effective against gram-negative and gram-positive bacteria. It is resistant to a variety of β-lactamases, and is effective against some naturally resistant bacterial species such as Pseudomonas aeruginosa, Staphylococcus aureus, and Streptococcus pneumoniae. Cefepime HCl is more soluble compared to the pure Cefepime. Both forms have similar potencies and both are suitable for microbiology use. However, Cefepime HCl can be easier to work with because of its higher aqueous solubility.
Cephalosporins are a type of β-lactam antibiotic consisting of a four-membered β-lactam ring bound to a six-membered dihydrothiazine ring. This two-ring system causes distortion of the β-lactam amide bond, resulting in decreased resonance stabilization and increased reactivity. β-lactams inhibit the formation of peptidoglycan cross-links within bacterial cell walls by targeting penicillin-binding proteins or PBPs. Consequently, the bacterial cell wall becomes weak and cytolysis occurs. Cephalosporins are less susceptible to β-lactamases than the penicillin β-lactam antibiotics.
Stock conc.: 10mg/mL, in PBS. Use immediately.
Store at -20oC