產品櫥窗
-
Tris Base
|
產品型號:101-77-86-1 商品規格: |
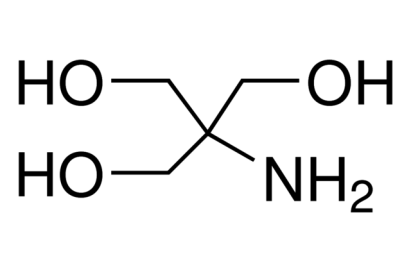 |
A component of molecular biology buffer, DNA electrophoresis. Synonym: THAM, Trizma® base, Trometamol
NH2C(CH2OH)3
M.W.: 121.14
Assay (By Titration): >99.0%
Heavy metals: 5ppm max.
pH (5% Soln. in water): 10.0-11.5
Solubility (40% Soln. in water): Clear and Colorless
White Crystalline Powder.
Tris is the most commonly used buffer in biological research. One of the most important applications is the use as an electrophoresis buffer for polyacrylamide and agarose gel electrophoresis, respectively. Tris should not be used at pH values under pH 7.2 or above pH 9.0. The pH value of a Tris buffer strongly depends on the temperature. Therefore, Tris buffers should be prepared at the temperature where it is used.
Good, N.E. et al. (1966) Biochemistry 5, 467-477 Hydrogen ion buffers for biological research.
Good, N.E. & Izawa, S. (1972) Methods Enzymol. 24, 53-68 Hydrogen ion buffers.
Ogden, R.C. & Adams, D.A. (1987) Methods Enzymol. 152, 61-87 Electrophoresis in agarose and acrylamide gels.
M.W.: 121.14
Assay (By Titration): >99.0%
Heavy metals: 5ppm max.
pH (5% Soln. in water): 10.0-11.5
Solubility (40% Soln. in water): Clear and Colorless
White Crystalline Powder.
Tris is the most commonly used buffer in biological research. One of the most important applications is the use as an electrophoresis buffer for polyacrylamide and agarose gel electrophoresis, respectively. Tris should not be used at pH values under pH 7.2 or above pH 9.0. The pH value of a Tris buffer strongly depends on the temperature. Therefore, Tris buffers should be prepared at the temperature where it is used.
Good, N.E. et al. (1966) Biochemistry 5, 467-477 Hydrogen ion buffers for biological research.
Good, N.E. & Izawa, S. (1972) Methods Enzymol. 24, 53-68 Hydrogen ion buffers.
Ogden, R.C. & Adams, D.A. (1987) Methods Enzymol. 152, 61-87 Electrophoresis in agarose and acrylamide gels.
Store at Room Temperature.
 Irritant
Irritant