產品櫥窗
-
Geldanamycin
|
產品型號:101-30562-34-6 商品規格: |
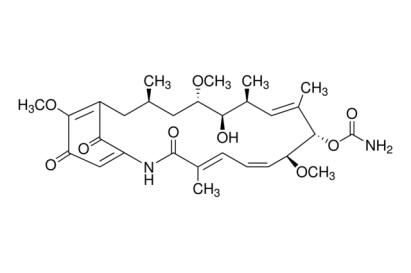 |
A potent antitumor antibiotic.
C29H40N2O9
M.W.: 560.6
Purity (TLC): >99%
Melting Point: 260~262℃
Soluble in DMSO.
Geldanamycin is a benzoquinone ansamycin antibiotic which binds to Hsp90 (Heat Shock Protein 90) and alters it function. Hsp90 is a highly conserved and very abundant protein in the cytosol of both eukaryotic and prokaryotic cells. Hsp90 plays a key role in regulating the physiology of cells exposed to environmental stress and in maintaining the malignant phenotype of tumor cells. HSP90 client proteins play important roles in the regulation of the cell cycle, cell growth, cell survival, apoptosis, and oncogenesis. Geldanamycin binds with a high affinity into the ATP binding pocket in Hsp90 and induces the degradation of proteins that require this chaperone for conformational maturation.
Whitsell, L., P.D. Sutphin, A.D. Lewis, P. Workman., Geldanamycin-Induced Association of Multiple Molecular Chaperone Proteins with Mutant p53 is Altered by Geldanamycin, an hsp90-Binding Agent., 1998., 18(3):, 1517-1524., 2. Lawson, B., J.W. Brewer, L.M Hendershot..
Gelanamycin, an hsp90/GRP94-Binding Drug, Induces Increased Transcription of Endoplasmic Reticulum (ER) Chaperones Via the ER Stress Pathway., Journal of Cellular Physiology., 1998., 174(2):, 170-178., 3. Joly, G.A., M. Ayres, R.G. Kilbourn..
Potent Inhibition of Inducible Nitric Oxide Synthase by Geldanamycin, A Tyrosine Kinase Inhibitor, in Endothelial, Smoothe Muscle Cells, and in Rat Aorta., FEBS Letters., 1997., 403(1):, 40-44., 4. Schulte, T.W., M.V. Blagosklonny, L. Romanova, J.F. Mushinski, B.P. Monia, J.F. Johnston..
Destabilization of Raf-1 by Geldanamycin Leads to Disruption of the Raf-1-MEK-Mitogen-Activated Protein Kinase Signalling Pathway., Molecular and Cellular Biology., 1996., 16(10):, 5839-5845..
M.W.: 560.6
Purity (TLC): >99%
Melting Point: 260~262℃
Soluble in DMSO.
Geldanamycin is a benzoquinone ansamycin antibiotic which binds to Hsp90 (Heat Shock Protein 90) and alters it function. Hsp90 is a highly conserved and very abundant protein in the cytosol of both eukaryotic and prokaryotic cells. Hsp90 plays a key role in regulating the physiology of cells exposed to environmental stress and in maintaining the malignant phenotype of tumor cells. HSP90 client proteins play important roles in the regulation of the cell cycle, cell growth, cell survival, apoptosis, and oncogenesis. Geldanamycin binds with a high affinity into the ATP binding pocket in Hsp90 and induces the degradation of proteins that require this chaperone for conformational maturation.
Whitsell, L., P.D. Sutphin, A.D. Lewis, P. Workman., Geldanamycin-Induced Association of Multiple Molecular Chaperone Proteins with Mutant p53 is Altered by Geldanamycin, an hsp90-Binding Agent., 1998., 18(3):, 1517-1524., 2. Lawson, B., J.W. Brewer, L.M Hendershot..
Gelanamycin, an hsp90/GRP94-Binding Drug, Induces Increased Transcription of Endoplasmic Reticulum (ER) Chaperones Via the ER Stress Pathway., Journal of Cellular Physiology., 1998., 174(2):, 170-178., 3. Joly, G.A., M. Ayres, R.G. Kilbourn..
Potent Inhibition of Inducible Nitric Oxide Synthase by Geldanamycin, A Tyrosine Kinase Inhibitor, in Endothelial, Smoothe Muscle Cells, and in Rat Aorta., FEBS Letters., 1997., 403(1):, 40-44., 4. Schulte, T.W., M.V. Blagosklonny, L. Romanova, J.F. Mushinski, B.P. Monia, J.F. Johnston..
Destabilization of Raf-1 by Geldanamycin Leads to Disruption of the Raf-1-MEK-Mitogen-Activated Protein Kinase Signalling Pathway., Molecular and Cellular Biology., 1996., 16(10):, 5839-5845..
Store at -20℃
Handling: Store in Tightly Sealed Vial. Protect from Light.
Handling: Store in Tightly Sealed Vial. Protect from Light.